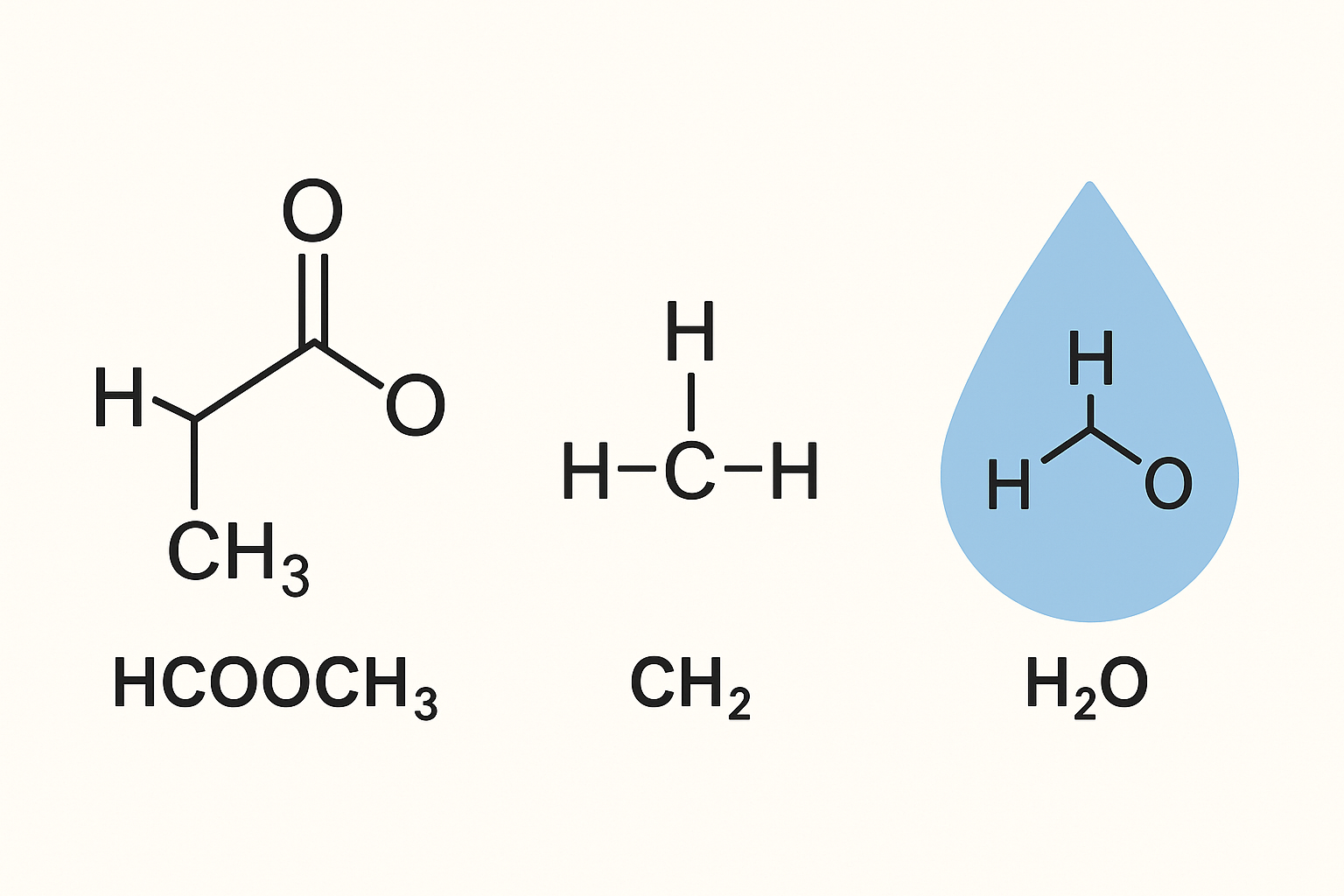
hcooch ch2 h2o Simple Guide for Students and Beginners
The term hcooch ch2 h2o may look like one chemical formula, it is not one single compound, it is a short way to describe a group of molecules that often take part in the same reaction, these molecules are TechStudify.com
-
a formate ester often called methyl formate
-
a CH2 unit also called a methylene group
-
water written as H2O
This guide explains each part in very simple words, it also explains the reaction that happens when these substances meet, it covers the properties uses and safety steps in an easy to understand way
What hcooch ch2 h2o Means
This expression shows three things that appear together in many chemistry topics
Formate ester
The part written as hcooch usually points to a formate ester, a common one is methyl formate, it has a carbonyl group that reacts fast with water
CH2 group
The CH2 group is a small part of bigger molecules, it is often found inside long carbon chains, it does not exist alone in normal conditions, it helps build many organic structures
Water
Water helps reactions happen, it can act as a solvent, it can also act as a reactant, it can break ester bonds during a process called hydrolysis
Simple Structures
Below is a quick look at the main structures in this system
Methyl formate
A small organic ester, it has a carbonyl carbon that reacts with water
CH2 unit
A simple two hydrogen one carbon group inside many molecules, it can link other groups together
Water
A very common liquid used in chemical reactions and living systems
Main Reaction of hcooch ch2 h2o
The most important reaction in this system is ester hydrolysis, this means that water breaks an ester into an acid and an alcohol
General reaction
An ester plus water gives a carboxylic acid plus an alcohol
Example reaction
Methyl formate plus water gives formic acid plus methanol
This shows how the ester group splits and forms two new products
Steps in simple form
-
Water attacks the carbonyl carbon
-
A short lived shape forms
-
The bond to the alcohol part breaks
-
The final form is an acid and an alcohol
Properties of the System
These substances have clear physical and chemical traits, below are simple points to understand them
Solubility
-
Water mixes with methanol
-
Formic acid mixes well with water
-
Methyl formate mixes less but still works in water based reactions
Stability
-
Temperature can change the reaction speed
-
Acid or base conditions can make hydrolysis faster
-
Water amount changes reaction progress
Boiling points Table
| Substance | Boiling point |
|---|---|
| Methyl formate | 31 C |
| Methanol | 64.7 C |
| Formic acid | 100.8 C |
| Water | 100 C |
Why This System Is Useful in Labs
The mix of hcooch ch2 h2o helps teach basic organic chemistry
It shows
-
Carbonyl reactions
-
Nucleophilic attack
-
Breaking and forming bonds
-
Use of acid and base
It helps with
-
Learning hydrolysis
-
Studying reaction speed
-
Understanding functional groups
Industrial and Daily Life Uses
This system is more than a school topic, it is used in many industries
Uses of methyl formate
-
Solvents in coatings and glues
-
Foam production
-
Making formamide and related chemicals
Uses of formic acid
-
Leather tanning
-
Rubber processing
-
Textile dye work
-
Silage treatment in farming
Uses of methanol
-
Fuel additives
-
Antifreeze
-
Making biodiesel
-
Making many organic products
Uses of CH2 units
-
Building polymers
-
Forming long chains
-
Shaping materials in industry
Environmental Importance
This system supports green chemistry ideas
Biodegradable nature
-
Formic acid breaks down easily
-
Methanol does not stay long in nature
-
Formate esters are not long lasting pollutants
Water as a safe medium
Water is safe and does not create toxic waste, this makes reactions cleaner
Space chemistry
Methyl formate has been found in space, scientists study it to learn how organic molecules form in stars and dust clouds
Safety Steps
These chemicals are simple yet they still need safe handling
Risks of each part
Methyl formate
-
Flammable
-
Can irritate the nose and eyes
Formic acid
-
Can burn skin
-
Strong smell
-
Needs careful storage
Methanol
-
Very toxic to drink
-
Can harm eyes and organs
-
Flammable
Basic safety rules
-
Wear gloves goggles and a lab coat
-
Work in a place with good airflow
-
Keep chemicals in labeled containers
-
Do not breathe vapors
-
Do not work near fire
Storage rules
-
Keep away from heat
-
Use sealed containers
-
Keep acids in safe chemical cabinets
If a spill happens
-
Move away from the area
-
Wear safety gear
-
Absorb liquid with safe material
-
Clean and dispose safely
Frequently Asked Questions
Is hcooch ch2 h2o one molecule?
No it is a short way to show a group of chemicals in one system
Is CH2 stable alone?
No it only exists inside larger molecules
Why is water needed?
Water helps break ester bonds and makes the reaction move
Is the reaction reversible?
Yes ester formation and ester breaking go both ways
Is this system safe for the environment?
Yes it breaks down into simple natural products